
Chemistry, 11.07.2019 07:30 djdkfkfkckmfmf7724
Amixture contains only nacl and al2(so4)3. a 1.45 g sample of the mixture is dissolved in water, and an excess of naoh is added, producing a precipitate of al(oh)3. the precipitate is filtered, dried, and weighed. the mass of the precipitate is 0.495 g. what is the mass percent of al2(so4)3 in the sample?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

You know the right answer?
Amixture contains only nacl and al2(so4)3. a 1.45 g sample of the mixture is dissolved in water, and...
Questions

Mathematics, 27.06.2019 01:00

Social Studies, 27.06.2019 01:00


English, 27.06.2019 01:00

Biology, 27.06.2019 01:00

Advanced Placement (AP), 27.06.2019 01:00



Mathematics, 27.06.2019 01:00


Biology, 27.06.2019 01:00





Social Studies, 27.06.2019 01:00


Social Studies, 27.06.2019 01:00

History, 27.06.2019 01:00

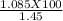 =74.8 %.
=74.8 %.

