
Chemistry, 11.07.2019 07:30 emilybutler
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decomposed the sulfur dioxide produced 3.49 g oxygen and 3.50 g sulfur, while the sulfur trioxide produced 6.75 g oxygen and 4.50 g sulfur. calculate the mass of oxygen per gram of sulfur for each sample and show that these results are consistent with the law of multiple proportions.

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:02
What is the partial pressure of ethane, pethane, in the flask?
Answers: 1

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decompose...
Questions

Arts, 14.09.2021 09:00


Mathematics, 14.09.2021 09:00




Mathematics, 14.09.2021 09:00

Mathematics, 14.09.2021 09:00



Biology, 14.09.2021 09:00

Computers and Technology, 14.09.2021 09:10

Mathematics, 14.09.2021 09:10


Chemistry, 14.09.2021 09:10


Mathematics, 14.09.2021 09:10


Geography, 14.09.2021 09:10

Mathematics, 14.09.2021 09:10

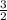 O₂
O₂

