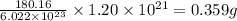Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

You know the right answer?
What is the mass, in grams, of 1.20×1021 molecules of aspirin, c9h8o4?...
Questions


Mathematics, 26.02.2021 01:10




Biology, 26.02.2021 01:10




Mathematics, 26.02.2021 01:10

Mathematics, 26.02.2021 01:10

Biology, 26.02.2021 01:10

History, 26.02.2021 01:10


Geography, 26.02.2021 01:10

Mathematics, 26.02.2021 01:10




Mathematics, 26.02.2021 01:10


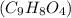 = 180.16 g/mol
= 180.16 g/mol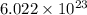 number of molecules are contained in 1 mole of a compound
number of molecules are contained in 1 mole of a compound