
Chemistry, 11.07.2019 07:00 kaylamaisonettt
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 4.15 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 4.15 ∘c. what is the molal concentration...
Questions






Mathematics, 21.07.2019 16:30





Mathematics, 21.07.2019 16:30


English, 21.07.2019 16:30

English, 21.07.2019 16:30

Social Studies, 21.07.2019 16:30


Mathematics, 21.07.2019 16:30

History, 21.07.2019 16:30

Mathematics, 21.07.2019 16:30

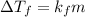 -(1)
-(1)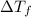 is depression of freezing point,
is depression of freezing point, 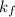 is freezing point depression constant and
is freezing point depression constant and 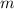 is molality.
is molality.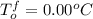 (given)
(given)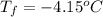 (given)
(given)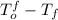
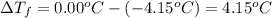
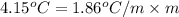
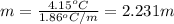
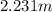 .
.

