
Chemistry, 11.07.2019 07:00 baeethtsadia
Acommon anesthetic in dentistry known as nitronox consists of a mixture of dinitrogen oxide, n2o, and oxygen gas, o2, which is administered through an inhaler over the nose. if the anesthetic mixture has a total pressure of 740 mmhg, and the partial pressure of the oxygen is 370 mmhg, what is the partial pressure, in torr, of the dinitrogen oxide?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3


Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1
You know the right answer?
Acommon anesthetic in dentistry known as nitronox consists of a mixture of dinitrogen oxide, n2o, an...
Questions

History, 11.12.2021 05:40

Mathematics, 11.12.2021 05:40

Mathematics, 11.12.2021 05:40

Social Studies, 11.12.2021 05:40

Mathematics, 11.12.2021 05:40


English, 11.12.2021 05:40


Health, 11.12.2021 05:40

Biology, 11.12.2021 05:40

Biology, 11.12.2021 05:40

Mathematics, 11.12.2021 05:40

Mathematics, 11.12.2021 05:40







Biology, 11.12.2021 05:50

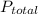 ) is sum of partial pressure of N₂O (
) is sum of partial pressure of N₂O (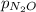 ) and partial pressure of O₂, (
) and partial pressure of O₂, (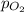 ).
).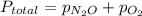
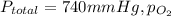 =370 mmHg
=370 mmHg

