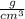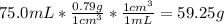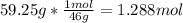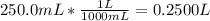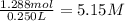Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
Asolution of ethanol (c2h5oh) in water is prepared by dissolving 75.0 ml of ethanol (density 0.79 g/...
Questions






Mathematics, 14.05.2021 18:20

Biology, 14.05.2021 18:20

Mathematics, 14.05.2021 18:20

Mathematics, 14.05.2021 18:20

Mathematics, 14.05.2021 18:20



History, 14.05.2021 18:20




Mathematics, 14.05.2021 18:20

Mathematics, 14.05.2021 18:20


Mathematics, 14.05.2021 18:20