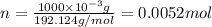An alka-seltzer tablet contains 324 mg of aspirin (c9h8o4), 1904 mg of nahco3, and 1000. mg of citric acid (h3c6h5o7). (the last two compounds react with each other to provide the "fizz," bubbles of co2, when the tablet is put into water.) calculate the amount (moles) of each substance in the tablet.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

You know the right answer?
An alka-seltzer tablet contains 324 mg of aspirin (c9h8o4), 1904 mg of nahco3, and 1000. mg of citri...
Questions

Biology, 23.09.2020 18:01









Mathematics, 23.09.2020 18:01






History, 23.09.2020 18:01



Mathematics, 23.09.2020 18:01

Biology, 23.09.2020 18:01

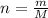
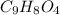 and molar mass of aspirin is 180.157 g/mol, thus, number of moles will be:
and molar mass of aspirin is 180.157 g/mol, thus, number of moles will be: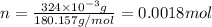
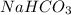 and molar mass is 84.007 g/mol, thus, number of moles will be:
and molar mass is 84.007 g/mol, thus, number of moles will be: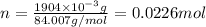
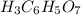 and molar mass is 192.124 g/mol, thus, number of moles will be:
and molar mass is 192.124 g/mol, thus, number of moles will be: