
10.00 ml of the final acid solution is reacted with excess barium chloride to produce a precipitate of barium sulfate (fw: 233.4 g/mol). the dry solid weighs 0.397 g. use this mass and the dilution volumes to calculate the actual molarity of the sulfuric acid in the initial solution. (previous question ask: 10.00 ml of approximately 6 m sulfuric acid is transferred to a 100ml volumetric flask and diluted to the mark with distilled water and mixed. then 10.00 ml of this solution was further diluted to 100 ml. the molarity of the final solution was 0.06 m).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
10.00 ml of the final acid solution is reacted with excess barium chloride to produce a precipitate...
Questions


Mathematics, 21.04.2020 20:10




Mathematics, 21.04.2020 20:10

Mathematics, 21.04.2020 20:10


Mathematics, 21.04.2020 20:10

Spanish, 21.04.2020 20:10




Biology, 21.04.2020 20:10






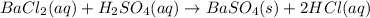
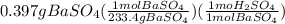
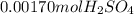
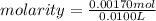
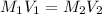
![Y=\frac{0.170M*100mL}{10.0mL}Y = 1.70MLet's do the similar calculations to find out the actual molarity of the original acid solution. Let's say the molarity of the original acid solution is X. 10.0 mL of it were taken and diluted to 100 mL on adding water. The molarity is 1.70M as is calculated in the above step. Let's plug in the values in the molarity equation again to solve it for X as:X(10.0mL) = 1.70M(100mL)[tex]X=\frac{1.70M*100mL}{10.0mL}](/tpl/images/0073/9434/d3677.png)



