Chemistry, 10.07.2019 16:30 jmurguia888
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass of 51.94051 amu. 9.500% of x has a mass of 52.94065 amu. 2.360% of x has a mass of 53.93888 amu. what is the average atomic mass of element x? express your answer numerically to four significant figures. view available hint(s)

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass...
Questions

Mathematics, 20.09.2020 05:01



Chemistry, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

Medicine, 20.09.2020 05:01


History, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


History, 20.09.2020 05:01

French, 20.09.2020 05:01

Health, 20.09.2020 05:01

History, 20.09.2020 05:01


English, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

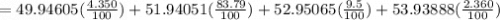
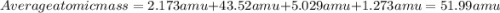
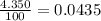
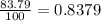
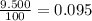
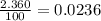
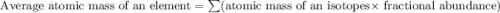
![A=\sum[(49.94605\times 0.0435)+(51.94051\times 0.8379)+(52.94065\times 0.095)+(53.93888\times 0.0236)]](/tpl/images/0073/8885/6e58e.png)