
Chemistry, 10.07.2019 15:00 hannahbanana2000
Which element does lithium (li) share more properties with, beryllium (be) or sodium (na)? lithium has more common properties with beryllium than sodium, because they have the same number of electron shells. lithium has more common properties with sodium than beryllium, because they have the same number of valence electrons. lithium has more common properties with beryllium than sodium, because they have the same number of valence electrons. lithium has more common properties with sodium than beryllium, because they have the same number of electron shells.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
Which element does lithium (li) share more properties with, beryllium (be) or sodium (na)? lithium h...
Questions

History, 19.04.2020 03:26


Mathematics, 19.04.2020 03:26

Mathematics, 19.04.2020 03:26


Mathematics, 19.04.2020 03:26

Physics, 19.04.2020 03:26

Mathematics, 19.04.2020 03:26

Mathematics, 19.04.2020 03:26








Health, 19.04.2020 03:44

Mathematics, 19.04.2020 03:44


Mathematics, 19.04.2020 03:44

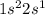 . This element belongs to Group 1 of the periodic table.
. This element belongs to Group 1 of the periodic table.

