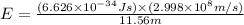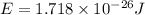Chemistry, 01.10.2019 20:30 kyramks421
Calculate the energy of a photon of wavelength 11.56 meters. (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108 m/s)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Calculate the energy of a photon of wavelength 11.56 meters. (planck’s constant is 6.626 x 10-34 jou...
Questions

Mathematics, 04.07.2019 05:00



History, 04.07.2019 05:00


History, 04.07.2019 05:00


History, 04.07.2019 05:00

Mathematics, 04.07.2019 05:00

Social Studies, 04.07.2019 05:00


Mathematics, 04.07.2019 05:00

Mathematics, 04.07.2019 05:00

Social Studies, 04.07.2019 05:00



Biology, 04.07.2019 05:00

Chemistry, 04.07.2019 05:00

Social Studies, 04.07.2019 05:00

History, 04.07.2019 05:00

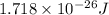
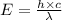
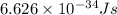
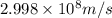
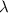 = wavelength = 11.56 m
= wavelength = 11.56 m