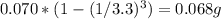In this experiment, 0.070 g of caffeine is dissolved in 4.0 ml of water. the caffeine is then extracted from the aqueous solution three times, each time using 2.0 ml fresh methylene chloride. calculate the total amount of caffeine that can be extracted into the three portions of methylene chloride (caffeine has a distribution coefficient of 4.6 between methylene chloride and water).

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 16:00
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a) 3.4 mol h2so4 b) 6.8 mol h2so4 c) 10.2 mol h2so4 d) 13.6 mol h2so4 a) 3.4 mol h2so4
Answers: 1

Chemistry, 23.06.2019 22:20
1.50 × 104 j of energy is transferred thermally into a huge tank filled with liquid water. the water temperature remains constant at 10.0 ∘c during the process.part aby how much does the entropy of the water change?
Answers: 1

Chemistry, 24.06.2019 00:20
How do you calculate percentage yield? what is the definition of percentage yield?
Answers: 1

Chemistry, 24.06.2019 01:30
What is released in the process of an atoms moving from an excited level to a lower energy level
Answers: 2
You know the right answer?
In this experiment, 0.070 g of caffeine is dissolved in 4.0 ml of water. the caffeine is then extrac...
Questions



Advanced Placement (AP), 13.04.2021 18:30


Mathematics, 13.04.2021 18:30





Mathematics, 13.04.2021 18:30

History, 13.04.2021 18:30

Mathematics, 13.04.2021 18:30



Mathematics, 13.04.2021 18:30


Mathematics, 13.04.2021 18:30

History, 13.04.2021 18:30


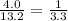 .
.