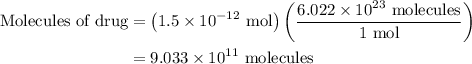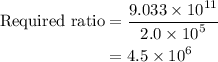Amedical lab is testing a new anticancer drug on cancer cells. the drug stock solution concentration is 1.5×10−9m, and 1.00 ml of this solution will be delivered to a dish containing 2.0×105 cancer cells in 5.00 ml of aqueous fluid. what is the ratio of drug molecules to the number of cancer cells in the dish?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1
You know the right answer?
Amedical lab is testing a new anticancer drug on cancer cells. the drug stock solution concentration...
Questions

Geography, 13.12.2021 15:00



Biology, 13.12.2021 15:00


English, 13.12.2021 15:00


French, 13.12.2021 15:00

English, 13.12.2021 15:00

Social Studies, 13.12.2021 15:00

Chemistry, 13.12.2021 15:00


World Languages, 13.12.2021 15:00

Biology, 13.12.2021 15:00

Chemistry, 13.12.2021 15:00

SAT, 13.12.2021 15:10

English, 13.12.2021 15:10


English, 13.12.2021 15:10

 .
.
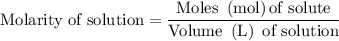 …… (1)
…… (1)
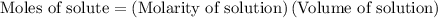 …… (2)
…… (2)
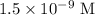 for molarity of solution and 1.00 mL for volume of solution in equation (2) to calculate moles of drug.
for molarity of solution and 1.00 mL for volume of solution in equation (2) to calculate moles of drug.
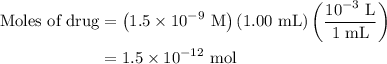
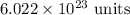 . These units can vary from question to question and can be atoms, molecules, or formula units. Since one mole of drug also contains
. These units can vary from question to question and can be atoms, molecules, or formula units. Since one mole of drug also contains 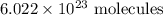 , number of molecules of drug present in
, number of molecules of drug present in 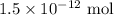 can be calculated as follows:
can be calculated as follows: