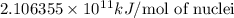Chemistry, 10.07.2019 12:30 janicemaxwell123
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and neutrons if an aluminum-27 atom has a mass of 26.9815386 amu? (the mass of an electron is 5.485799×10−4 amu, the mass of a proton is 1.0072765 amu, and the mass of a neutron is 1.0086649 amu.) express your answer using six significant figures. g?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and ne...
Questions





Health, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01


English, 20.10.2020 04:01

Advanced Placement (AP), 20.10.2020 04:01

English, 20.10.2020 04:01

History, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01


English, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01


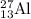 nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from
nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from 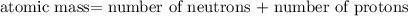 .
. 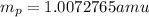 and
and 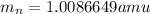 , we can calculate the mass of the isotope.
, we can calculate the mass of the isotope.
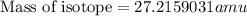
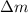 = mass of isotope - atomic mass.
= mass of isotope - atomic mass.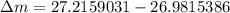

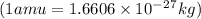



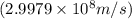


 and converting individual particles into moles, we need to multiply it by avagadro's number that is
and converting individual particles into moles, we need to multiply it by avagadro's number that is  .
.