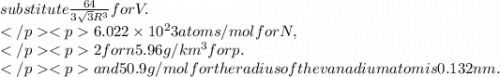Chemistry, 10.07.2019 08:30 dazesreplayy2451
Calculate the radius of a vanadium atom, given that v has a bcc crystal structure, a density of 5.96 g/cm3 , and an atomic weight of 50.9 g/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Calculate the radius of a vanadium atom, given that v has a bcc crystal structure, a density of 5.96...
Questions

Chemistry, 05.02.2021 18:20

Mathematics, 05.02.2021 18:20


Mathematics, 05.02.2021 18:20


Social Studies, 05.02.2021 18:20


Mathematics, 05.02.2021 18:20

Mathematics, 05.02.2021 18:20

Mathematics, 05.02.2021 18:20




Mathematics, 05.02.2021 18:20


English, 05.02.2021 18:20

Advanced Placement (AP), 05.02.2021 18:20



History, 05.02.2021 18:20