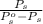An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, ch3oh. the vapor pressure of pure methanol at 20°c is 89 mmhg. if 6.0 g of urea is mixed with 39.8 g of methanol, calculate the vapor pressure of the methanol solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

You know the right answer?
An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, c...
Questions

Social Studies, 14.12.2019 13:31



Chemistry, 14.12.2019 13:31

French, 14.12.2019 13:31




Mathematics, 14.12.2019 13:31


Biology, 14.12.2019 13:31

History, 14.12.2019 13:31

Biology, 14.12.2019 13:31

Biology, 14.12.2019 13:31

Mathematics, 14.12.2019 13:31


History, 14.12.2019 13:31

English, 14.12.2019 13:31