Chemistry, 10.07.2019 08:30 tybreyonnaHco7855
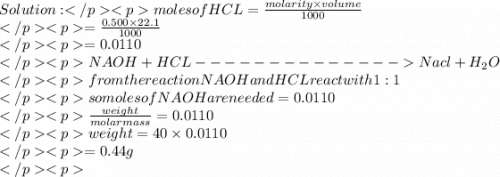
Asample contains both naoh and nacl. 0.500 g of this sample was dissolved in water to make a 20.0 ml solution and then this solution was titrated by 0.500 mol/l hcl solution. if 22.1 ml of hcl was used to reach the end point, what is the mass % of naoh in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Asample contains both naoh and nacl. 0.500 g of this sample was dissolved in water to make a 20.0 ml...
Questions



Advanced Placement (AP), 02.09.2021 22:50

Mathematics, 02.09.2021 22:50


Mathematics, 02.09.2021 22:50


Mathematics, 02.09.2021 22:50




Mathematics, 02.09.2021 22:50

Mathematics, 02.09.2021 22:50




Spanish, 02.09.2021 22:50

Health, 02.09.2021 22:50

Mathematics, 02.09.2021 22:50

Biology, 02.09.2021 22:50