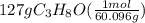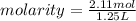Chemistry, 10.07.2019 07:30 sduhaime1974
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropanol is dissolved in water. the volume of the solution is 1,250 milliliters. what is the molarity of the solution? refer to the periodic table to you answer. express your answer to three significant figures. the molarity of the solution is m.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropan...
Questions


Mathematics, 20.12.2019 01:31

Social Studies, 20.12.2019 01:31

English, 20.12.2019 01:31




English, 20.12.2019 01:31



History, 20.12.2019 01:31

Mathematics, 20.12.2019 01:31




Computers and Technology, 20.12.2019 01:31


Physics, 20.12.2019 01:31


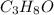 = 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol
= 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol