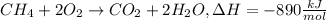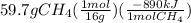Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3
You know the right answer?
Ch4 + 2o2 → co2 + 2h2o, δh = -890 kj/mol how much energy is released when 59.7 grams of methane (ch4...
Questions




Mathematics, 11.12.2021 21:20


Mathematics, 11.12.2021 21:20



Computers and Technology, 11.12.2021 21:20


History, 11.12.2021 21:20

History, 11.12.2021 21:20


Computers and Technology, 11.12.2021 21:20

Mathematics, 11.12.2021 21:20

Mathematics, 11.12.2021 21:20

Mathematics, 11.12.2021 21:20

History, 11.12.2021 21:20

Arts, 11.12.2021 21:20

Mathematics, 11.12.2021 21:20