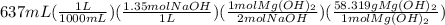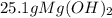Chemistry, 10.07.2019 07:30 andybiersack154
Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 + 2nacl. suppose the reaction begins with 637 milliliters of 1.35 m sodium hydroxide solution and excess magnesium hydroxide. what is the theoretical yield of magnesium hydroxide if the resulting solution has a volume of 2.82 liters? use the periodic table and the polyatomic ion resource. the mass of magnesium hydroxide formed is grams.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 +...
Questions




















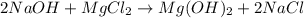
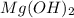 = 24.305 + 2( 15.999 + 1.008)
= 24.305 + 2( 15.999 + 1.008)