Chemistry, 10.07.2019 06:00 Meap12345678910
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration...
Questions


Mathematics, 22.01.2021 20:40

Chemistry, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40


Arts, 22.01.2021 20:40


Mathematics, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40




Mathematics, 22.01.2021 20:40


English, 22.01.2021 20:40

Mathematics, 22.01.2021 20:40

 can be calculated as follows:
can be calculated as follows: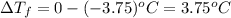

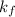 is freezing point depression constant.
is freezing point depression constant.