
Chemistry, 10.07.2019 06:00 iicekingmann
When silver crystallizes, it forms face-centered cubic cells. the unit cell edge length is 408.7 pm. calculate the density of silver in g/cm3?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 11:00
Intermolecular forces. question i need with: the only intermolecular forces that affect non polar molecules are forces.
Answers: 2


Chemistry, 23.06.2019 16:10
What type of reaction is shown below? check all that apply. agno3(aq) + nacl(aq) → nano3(aq) + agcl(s) i synthesis decomposition combustion i single replacement double replacement done
Answers: 2

Chemistry, 23.06.2019 18:20
Ahydrogen electron is elevated from level 1 to level 2. another electron is elevated from level 2 to level 4. the transition requiring the greatest energy change is?
Answers: 1
You know the right answer?
When silver crystallizes, it forms face-centered cubic cells. the unit cell edge length is 408.7 pm....
Questions


Computers and Technology, 04.07.2019 20:30






Computers and Technology, 04.07.2019 20:30



Physics, 04.07.2019 20:30

Computers and Technology, 04.07.2019 20:30







English, 04.07.2019 20:30

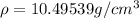
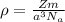
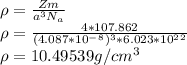
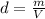
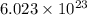 atoms, thus, mass can be calculated as follows:
atoms, thus, mass can be calculated as follows: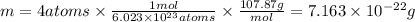 .
.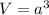
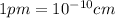
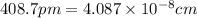
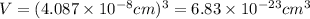
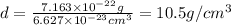
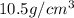 .
.

