
Chemistry, 10.07.2019 06:00 xbeatdroperzx
Phenol, c6h5oh, is a stronger acid than methanol, ch3oh, even though both contain an o]h bond. draw the structures of the anions resulting from loss of h1 from phenol and methanol, and use resonance structures to explain the difference in acidity.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2


Chemistry, 24.06.2019 03:30
Identify the reagents represented by the letters in the above reaction scheme. (enter your answer as a string of letters without punctuation, e.g. abcd.)
Answers: 1
You know the right answer?
Phenol, c6h5oh, is a stronger acid than methanol, ch3oh, even though both contain an o]h bond. draw...
Questions



Mathematics, 04.06.2020 03:58


Mathematics, 04.06.2020 03:58


History, 04.06.2020 03:58




Social Studies, 04.06.2020 03:58

Mathematics, 04.06.2020 03:58




Mathematics, 04.06.2020 03:59




Mathematics, 04.06.2020 03:59

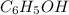 and methanol,
and methanol, 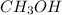 both are alcohols that contain an
both are alcohols that contain an 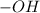 group attached to carbon atom.
group attached to carbon atom. from phenol, it forms phenoxide anion and due to presence of double bond in the benzene ring the negative charge on the oxygen atom (which represents electrons) will resonate with double bonds of benzene ring as shown in the image. The resonance-stabilized phenoxide ion is more stable. Whereas when methanol lose 1
from phenol, it forms phenoxide anion and due to presence of double bond in the benzene ring the negative charge on the oxygen atom (which represents electrons) will resonate with double bonds of benzene ring as shown in the image. The resonance-stabilized phenoxide ion is more stable. Whereas when methanol lose 1 ![Phenol, c6h5oh, is a stronger acid than methanol, ch3oh, even though both contain an o]h bond. draw](/tpl/images/0072/2100/80d06.jpg)


