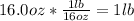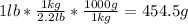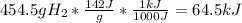Chemistry, 10.07.2019 06:00 coryintheswamp
Combustion of hydrogen releases 142 j/g of hydrogen reacted. how many kj of energy are released by the combustion of 16.0 oz of hydrogen? (1 lb = 16 oz; 1 kg = 2.2 lb)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Combustion of hydrogen releases 142 j/g of hydrogen reacted. how many kj of energy are released by t...
Questions

Mathematics, 17.10.2020 18:01


Mathematics, 17.10.2020 18:01

Mathematics, 17.10.2020 18:01


Biology, 17.10.2020 18:01

Business, 17.10.2020 18:01

Arts, 17.10.2020 18:01