

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
An unknown liquid is composed of 34.31% c, 5.28% h, and 60.41% i. the molecular weight is 210.06 amu...
Questions

Mathematics, 21.08.2019 07:30


English, 21.08.2019 07:30


Mathematics, 21.08.2019 07:30

History, 21.08.2019 07:30


Computers and Technology, 21.08.2019 07:30


Mathematics, 21.08.2019 07:30


Social Studies, 21.08.2019 07:30



Mathematics, 21.08.2019 07:30

Social Studies, 21.08.2019 07:30

Biology, 21.08.2019 07:30



Biology, 21.08.2019 07:30

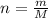
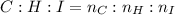
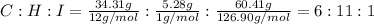
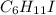 .
.

