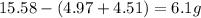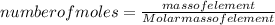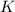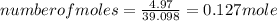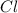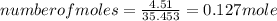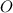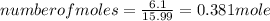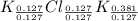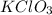Chemistry, 10.07.2019 05:30 geraldmorgan5580
A15.58 g sample of a compound contains 4.97 g potassium (k), 4.51 g chlorine (cl), and oxygen (o). calculate the empirical formula.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3


Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
A15.58 g sample of a compound contains 4.97 g potassium (k), 4.51 g chlorine (cl), and oxygen (o). c...
Questions

Social Studies, 27.07.2019 02:00

Social Studies, 27.07.2019 02:00


Biology, 27.07.2019 02:00

Computers and Technology, 27.07.2019 02:00


Computers and Technology, 27.07.2019 02:00

Computers and Technology, 27.07.2019 02:00


Mathematics, 27.07.2019 02:00

Mathematics, 27.07.2019 02:00






Computers and Technology, 27.07.2019 02:00

History, 27.07.2019 02:00

Social Studies, 27.07.2019 02:00