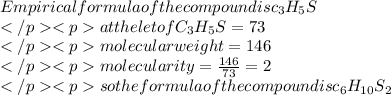Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
Acompound responsible for the odor of garlic has a molecular weight of 146 g/mol. a 0.650 g sample o...
Questions

Health, 09.01.2021 08:10

Mathematics, 09.01.2021 08:10


Chemistry, 09.01.2021 08:10



Mathematics, 09.01.2021 08:10

Spanish, 09.01.2021 08:10



Physics, 09.01.2021 08:10


Mathematics, 09.01.2021 08:20




Mathematics, 09.01.2021 08:20