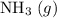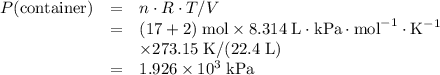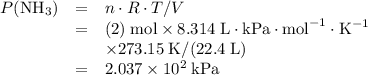Chemistry, 10.07.2019 05:30 sportsseolive4471
Avessel of volume 22.4 dm3 contains 20 mol h2 and 1 mol n2 ad 273.15 k initially. all of the nitrogen reacted with sufficient hydrogen to form nh3. calculate the total pressure and the partial pressure of each component in the final mixture at 273.15 k.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
Avessel of volume 22.4 dm3 contains 20 mol h2 and 1 mol n2 ad 273.15 k initially. all of the nitroge...
Questions



Advanced Placement (AP), 20.11.2020 22:10

Mathematics, 20.11.2020 22:10



World Languages, 20.11.2020 22:10


Chemistry, 20.11.2020 22:10

Geography, 20.11.2020 22:10


Mathematics, 20.11.2020 22:10

Social Studies, 20.11.2020 22:10



Mathematics, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10

English, 20.11.2020 22:10


Biology, 20.11.2020 22:10

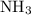 at a
at a 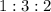 ratio:
ratio: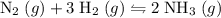
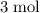 of hydrogen gas would have been consumed while
of hydrogen gas would have been consumed while 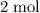 of ammonia would have been produced. The final mixture would therefore contain
of ammonia would have been produced. The final mixture would therefore contain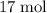 of
of 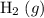 and
and