
Chemistry, 10.07.2019 05:30 anitadefrances
Use enthalpies of formation given in appendix c to calculate δh for the reaction br2(g)→2br(g), and use this value to estimate the bond enthalpy d(br−br).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
Use enthalpies of formation given in appendix c to calculate δh for the reaction br2(g)→2br(g), and...
Questions


Social Studies, 04.03.2021 23:20

Social Studies, 04.03.2021 23:20








Chemistry, 04.03.2021 23:30

Mathematics, 04.03.2021 23:30

Mathematics, 04.03.2021 23:30


Mathematics, 04.03.2021 23:30

English, 04.03.2021 23:30




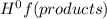 - ∑n(reactants)Δ
- ∑n(reactants)Δ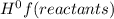
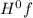 = enthalpy of formation
= enthalpy of formation


