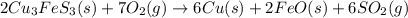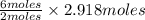Chemistry, 10.07.2019 03:30 astepania0003
Using the balanced chemical reaction below, how many grams of copper (cu) metal can be obtained from 1.00 kg of copper ore (cu3fes3)? 2cu3fes3 (s) + 7o2 (g) ; → 6cu (s) + 2feo (s) + 6so2 (g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Using the balanced chemical reaction below, how many grams of copper (cu) metal can be obtained from...
Questions

Biology, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01

Chemistry, 16.10.2020 03:01

Law, 16.10.2020 03:01


Computers and Technology, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

English, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01



Mathematics, 16.10.2020 03:01

SAT, 16.10.2020 03:01

History, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

Spanish, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01

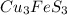 = 342.678 g/mole
= 342.678 g/mole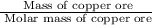 =
= 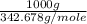 = 2.918 moles
= 2.918 moles