

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3
You know the right answer?
Calculate the mean free path of air molecules at 3.50 * 10-13 atm and 300 k. (this pressure is readi...
Questions




Mathematics, 11.03.2021 18:20


English, 11.03.2021 18:20

Mathematics, 11.03.2021 18:20

Geography, 11.03.2021 18:20

English, 11.03.2021 18:20

Computers and Technology, 11.03.2021 18:20

Biology, 11.03.2021 18:20


Mathematics, 11.03.2021 18:20



Computers and Technology, 11.03.2021 18:20

Social Studies, 11.03.2021 18:20


Mathematics, 11.03.2021 18:20

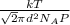 ---------(1)
---------(1)

