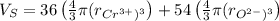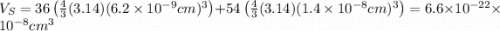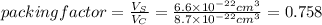Chemistry, 09.07.2019 22:00 nancye2008
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 nm. if the density of this material is 5.22 g/cm3, calculate its atomic packing factor. the atomic weights of cr

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1


Chemistry, 23.06.2019 15:20
How many stereoisomers will be formed from the addition of phenyllithium to this molecule?
Answers: 1
You know the right answer?
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 n...
Questions

English, 25.09.2020 15:01



Mathematics, 25.09.2020 15:01

Mathematics, 25.09.2020 15:01

Biology, 25.09.2020 15:01


Mathematics, 25.09.2020 15:01

History, 25.09.2020 15:01


Mathematics, 25.09.2020 15:01

Health, 25.09.2020 15:01






Mathematics, 25.09.2020 15:01


Mathematics, 25.09.2020 15:01

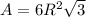
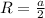
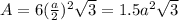
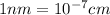
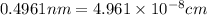
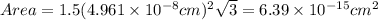
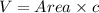
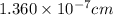
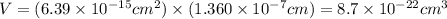
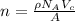
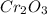 is 151.99 g/mol.
is 151.99 g/mol.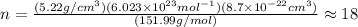
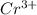 and
and 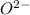 is 62 pm and 140 pm respectively.
is 62 pm and 140 pm respectively. 
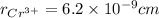
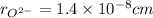
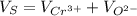
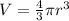 ,
,