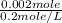Chemistry, 09.07.2019 21:30 brianna4455
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of it. the stockroom provides a 0.2 mol/l hcl solution as the titrant. what volume of hcl (in ml) is needed to reach the equivalence (stoichiometric) point if your unknown compound is naoh? naoh(aq) + hcl(aq) --> nacl(aq) + h2o(l)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of...
Questions

Mathematics, 30.08.2020 01:01



Chemistry, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Medicine, 30.08.2020 01:01


Mathematics, 30.08.2020 01:01

History, 30.08.2020 01:01

History, 30.08.2020 01:01

Computers and Technology, 30.08.2020 01:01




Business, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01


Physics, 30.08.2020 01:01

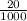 × 0.1
× 0.1