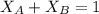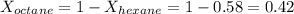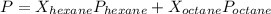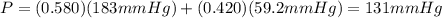Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
At a given temperature the vapor pressures of hexane and octane are 183 mmhg and 59.2 mmhg, respecti...
Questions

Biology, 17.10.2019 19:30



Mathematics, 17.10.2019 19:30

History, 17.10.2019 19:30

Mathematics, 17.10.2019 19:30



Mathematics, 17.10.2019 19:30

Mathematics, 17.10.2019 19:30

Arts, 17.10.2019 19:30



Biology, 17.10.2019 19:30




History, 17.10.2019 19:30

Advanced Placement (AP), 17.10.2019 19:30

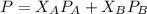
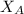 is mole fraction of A,
is mole fraction of A, 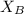 is mole fraction of B,
is mole fraction of B, 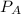 is partial pressure of A and
is partial pressure of A and 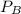 is partial pressure of B.
is partial pressure of B.