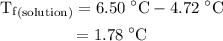Chemistry, 09.07.2019 21:00 genyjoannerubiera
Part a cyclohexane has a freezing point of 6.50 ∘c and a kf of 20.0 ∘c/m. what is the freezing point of a solution made by dissolving 0.925 g of biphenyl (c12h10) in 25.0 g of cyclohexane?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Part a cyclohexane has a freezing point of 6.50 ∘c and a kf of 20.0 ∘c/m. what is the freezing point...
Questions




Mathematics, 26.10.2019 05:43

Mathematics, 26.10.2019 05:43



Biology, 26.10.2019 05:43

Biology, 26.10.2019 05:43


Mathematics, 26.10.2019 05:43


Biology, 26.10.2019 05:43


Mathematics, 26.10.2019 05:43

Mathematics, 26.10.2019 05:43


Mathematics, 26.10.2019 05:43



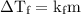 ...... (1)
...... (1)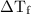 is the change in freezing point.
is the change in freezing point.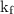 is the freezing point depression constant.
is the freezing point depression constant.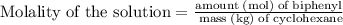 ……. (2)
……. (2)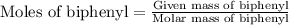 …… (3)
…… (3)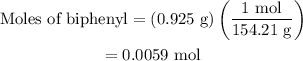

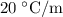 .
.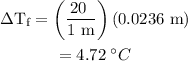
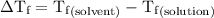 ...... (4)
...... (4)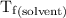 is the temperature of the solvent.
is the temperature of the solvent.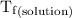 is the temperature of the solution.
is the temperature of the solution.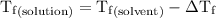 ……. (5)
……. (5)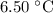 for
for 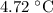 for
for