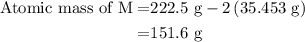Chemistry, 09.07.2019 21:00 balwinderdev
A0.999-g sample of a metal chloride, mcl2, is dissolved in water and treated with excess aqueous silver nitrate. the silver chloride that formed weighed 1.286 g. calculate the atomic mass of m.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
A0.999-g sample of a metal chloride, mcl2, is dissolved in water and treated with excess aqueous sil...
Questions

History, 11.04.2020 00:02


English, 11.04.2020 00:02


English, 11.04.2020 00:02


Mathematics, 11.04.2020 00:02

Mathematics, 11.04.2020 00:02

Mathematics, 11.04.2020 00:02


Mathematics, 11.04.2020 00:02

History, 11.04.2020 00:02



Mathematics, 11.04.2020 00:02


Physics, 11.04.2020 00:03

Mathematics, 11.04.2020 00:03


Mathematics, 11.04.2020 00:03

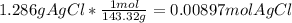

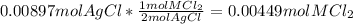
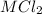 =0.999 g
=0.999 g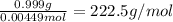
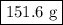 .
.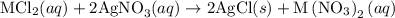
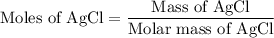 ...... (1)
...... (1)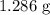 for mass of AgCl and
for mass of AgCl and 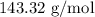 for molar mass of AgCl in equation (1).
for molar mass of AgCl in equation (1).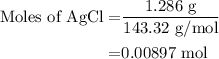
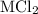 reacts with two moles of
reacts with two moles of 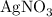 to form two moles of AgCl and one mole of
to form two moles of AgCl and one mole of 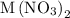 , So stoichiometric ratio between
, So stoichiometric ratio between 
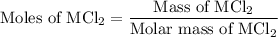 ...... (2)
...... (2)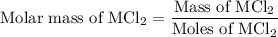 ...... (3)
...... (3)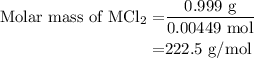
![\text{Molar mass of MCl}_2=\left[1\left(\text{Atomic mass of M}\right)+\\\\2\left(\text{Atomic mass of Cl}\right)\right]}](/tpl/images/0070/7913/9c07b.png) ....... (4)
....... (4)![\text{Atomic mass of M}=\left[\text{Molar mass of MCl}_2-2\left(\text{Atomic mass of Cl}\right)\right]](/tpl/images/0070/7913/5c489.png) ...... (5)
...... (5)