
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is dissolved in water. then all of the bromide ion is precipitated as agbr by the addition of an excess of silver nitrate. babr2(> 2agbr(s)+ba(no3)2(aq). in one experiment a 0.7207 g sample of the mixture resulted in 0.6226 g of agbr. determine the percent (by mass) of ba in the mixture

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is d...
Questions








World Languages, 26.08.2021 18:20


Mathematics, 26.08.2021 18:20

Engineering, 26.08.2021 18:20

Mathematics, 26.08.2021 18:20


Business, 26.08.2021 18:20

Mathematics, 26.08.2021 18:20

Biology, 26.08.2021 18:20



Business, 26.08.2021 18:20

English, 26.08.2021 18:20

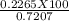 =31.42 %
.
=31.42 %
.

