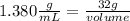Chemistry, 09.07.2019 17:30 raveransaw
If the density of corn syrup is 1.380 g/ml and a sample of corn syrup has a mass of 32 grams, what is the volume of corn syrup, in liters?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
If the density of corn syrup is 1.380 g/ml and a sample of corn syrup has a mass of 32 grams, what i...
Questions


History, 06.11.2019 18:31

Mathematics, 06.11.2019 18:31

Mathematics, 06.11.2019 18:31



Mathematics, 06.11.2019 18:31

History, 06.11.2019 18:31




Computers and Technology, 06.11.2019 18:31






Mathematics, 06.11.2019 18:31


Mathematics, 06.11.2019 18:31

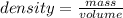
 Mass= 32 g
Mass= 32 g