
Chemistry, 09.07.2019 17:00 qudoniselmore0
In a lithium ion battery that is discharging to power a device, for every li+ that inserts into the lithium cobalt oxide electrode, a co4+ ion must be reduced to a co3+ ion in order to balance the charge. using the crc handbook of chemistry and physics or other standard reference, find the ionic radii of li+, co3+, and co4+. order these ions from largest to smallest.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
In a lithium ion battery that is discharging to power a device, for every li+ that inserts into the...
Questions






Mathematics, 16.01.2021 21:50

Mathematics, 16.01.2021 21:50

Spanish, 16.01.2021 21:50





English, 16.01.2021 21:50


French, 16.01.2021 21:50

Mathematics, 16.01.2021 21:50



Mathematics, 16.01.2021 21:50

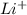 ,
, 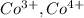 are 90 pm, 75 pm, 67 pm respectively from standard reference.
are 90 pm, 75 pm, 67 pm respectively from standard reference.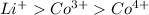 .
. ion is larger than the
ion is larger than the 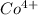 ion because as the positive charge increases on the ion then the size of the ion decreases.
ion because as the positive charge increases on the ion then the size of the ion decreases.

