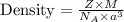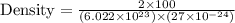Chemistry, 09.07.2019 17:00 jaallen3679
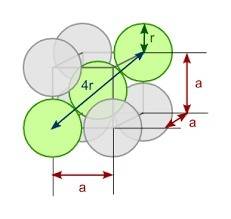
You discover a new alloy made up of cu and ni atoms. it has a bcc structure, where the ni atoms are located at the corners and the cu atoms are located in the center of each cell. the radius of the cu atoms is 0.13 nm and the radius of the ni atom is 0.15 nm. calculate the density of this structure. assume that cu has an atomic weight of 40 g/mol and ni has an atomic weight of 60 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
You discover a new alloy made up of cu and ni atoms. it has a bcc structure, where the ni atoms are...
Questions


Mathematics, 05.10.2019 15:40


Computers and Technology, 05.10.2019 15:40


Biology, 05.10.2019 15:40


English, 05.10.2019 15:40



Mathematics, 05.10.2019 15:40

History, 05.10.2019 15:40

Geography, 05.10.2019 15:40

Mathematics, 05.10.2019 15:40

English, 05.10.2019 15:40

History, 05.10.2019 15:40

Social Studies, 05.10.2019 15:40

Physics, 05.10.2019 15:40

Mathematics, 05.10.2019 15:40

History, 05.10.2019 15:40

 ) = 6.022 ×
) = 6.022 × 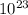
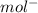
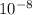 cm
cm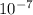 cm
cm =
=  = 27 ×
= 27 × 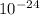

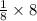 = 1atom of Ni and 1 atom of Cu
= 1atom of Ni and 1 atom of Cu