
Be sure to answer all parts. industrially, hydrogen gas can be prepared by combining propane gas (c3h8) with steam at about 400°c. the products are carbon monoxide (co) and hydrogen gas (h2). (a) write a balanced equation for the reaction. include phase abbreviations. (b) how many kilograms of h2 can be obtained from 8.31 × 103 kg of propane

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

You know the right answer?
Be sure to answer all parts. industrially, hydrogen gas can be prepared by combining propane gas (c3...
Questions

Geography, 10.02.2021 01:10

Mathematics, 10.02.2021 01:10

Health, 10.02.2021 01:10




Mathematics, 10.02.2021 01:10

Mathematics, 10.02.2021 01:10

Mathematics, 10.02.2021 01:10

Mathematics, 10.02.2021 01:10

English, 10.02.2021 01:10


English, 10.02.2021 01:10





Biology, 10.02.2021 01:10

Mathematics, 10.02.2021 01:10

Mathematics, 10.02.2021 01:10

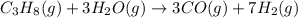


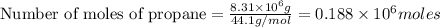
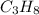 gives 7 moles of
gives 7 moles of 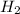
 moles of
moles of  of
of 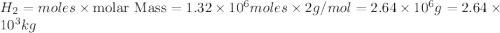
 kg of propane
kg of propane

