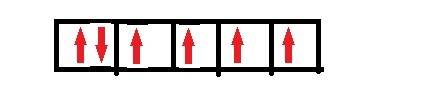
When transition metal atoms lose electrons to form ions, the electrons come first from the highest filled s atomic orbital. with this in mind , how many unpaired electrons are present in the fe2+ ion? hint: first write the outermost electron configuration of fe atom?

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
You know the right answer?
When transition metal atoms lose electrons to form ions, the electrons come first from the highest f...
Questions



History, 05.12.2019 20:31



Mathematics, 05.12.2019 20:31


History, 05.12.2019 20:31

Biology, 05.12.2019 20:31


Mathematics, 05.12.2019 20:31



Biology, 05.12.2019 20:31




Biology, 05.12.2019 20:31