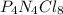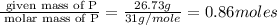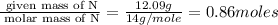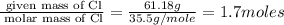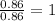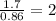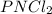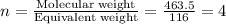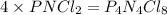“analysis of a compound of phosphorus nitrogen and chlorine show that it is 26.73% p and 12.09% n with cl accounting for the remainder. in a separate experiment, the molar mass of the compound was found to be 463.5 g/mol. determine the molecular formula of the compound.” !

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
“analysis of a compound of phosphorus nitrogen and chlorine show that it is 26.73% p and 12.09% n wi...
Questions

Mathematics, 03.07.2019 11:30

Biology, 03.07.2019 11:30


English, 03.07.2019 11:30


Mathematics, 03.07.2019 11:30




Mathematics, 03.07.2019 11:30



Spanish, 03.07.2019 11:30