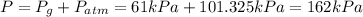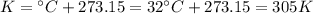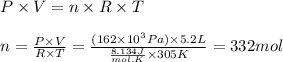Chemistry, 09.07.2019 15:30 josie17340
Larisa pumps up a soccer ball until it has a gauge pressure of 61 kilopascals. the volume of the ball is 5.2 liters. the air temperature is 32°c, and the outside air is at standard pressure. how many moles of air are in the ball?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Larisa pumps up a soccer ball until it has a gauge pressure of 61 kilopascals. the volume of the bal...
Questions

Mathematics, 08.12.2020 21:40




Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40

Social Studies, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40