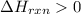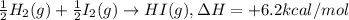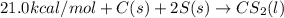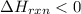Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2


Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
½h2(g) + ½i2(g) → hi(g), δh = +6.2 kcal/mole 21.0 kcal/mole + c(s) + 2s(s) → cs2(l) what type of rea...
Questions


Chemistry, 09.12.2019 21:31



Mathematics, 09.12.2019 21:31


Mathematics, 09.12.2019 21:31

English, 09.12.2019 21:31

History, 09.12.2019 21:31

Physics, 09.12.2019 21:31

Geography, 09.12.2019 21:31


Spanish, 09.12.2019 21:31

Spanish, 09.12.2019 21:31

Mathematics, 09.12.2019 21:31


Biology, 09.12.2019 21:31


English, 09.12.2019 21:31

History, 09.12.2019 21:31