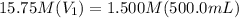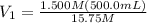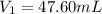Chemistry, 09.07.2019 13:30 genyjoannerubiera
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist with 10.00 l of 15.75 m perchloric acid solution to prepare the required solution. calculate the volume of concentrated acid required.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1

Chemistry, 23.06.2019 10:00
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
You know the right answer?
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist wi...
Questions

Mathematics, 23.03.2021 19:20

Mathematics, 23.03.2021 19:20

Chemistry, 23.03.2021 19:20

Mathematics, 23.03.2021 19:20


Mathematics, 23.03.2021 19:20

Biology, 23.03.2021 19:20


Social Studies, 23.03.2021 19:20

Mathematics, 23.03.2021 19:20



Mathematics, 23.03.2021 19:20

Mathematics, 23.03.2021 19:20

Spanish, 23.03.2021 19:20

English, 23.03.2021 19:20

Biology, 23.03.2021 19:20

Social Studies, 23.03.2021 19:20


Chemistry, 23.03.2021 19:20

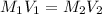
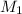 is the concentration of the concentrated solution and
is the concentration of the concentrated solution and 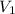 is it's volume.
is it's volume. 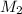 is the concentration of the diluted solution and
is the concentration of the diluted solution and 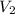 is it's volume. Let's plug in the values in the equation and solve it for
is it's volume. Let's plug in the values in the equation and solve it for