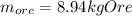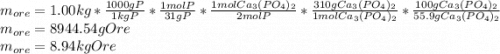Chemistry, 09.07.2019 13:00 deontehiggins42
Phosphorus is obtained primarily from ores containing calcium phosphate. part a if a particular ore contains 55.9 % calcium phosphate, what minimum mass of the ore must be processed to obtain 1.00 kg of phosphorus? express your answer with the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
Phosphorus is obtained primarily from ores containing calcium phosphate. part a if a particular ore...
Questions

History, 19.11.2020 20:10



Mathematics, 19.11.2020 20:10


English, 19.11.2020 20:10





Mathematics, 19.11.2020 20:10

Mathematics, 19.11.2020 20:10



Physics, 19.11.2020 20:10

Mathematics, 19.11.2020 20:10




History, 19.11.2020 20:10