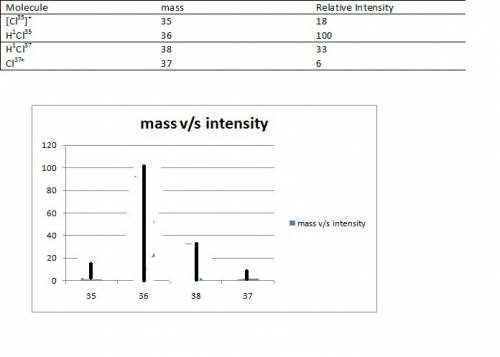
(7 pts) hydrogen and chlorine atoms react to form simple diatomic molecules in a 1: 1 ratio that is hcl. the natural abundances of the chlorine isotopes are 75.77% 35cl and 24.23% 37cl. the natural abundance of 1h is 99.985%, the natural abundance of 2h is 0.015% and there is only a trace (less than 0.001%) of 3h. (a) how many different peaks can hcl produce in a mass spectrometer and what is the mass number for each peak observed? (b) what is the relative abundance of each mass. (c) sketch the mass spectrometry analysis output (relative number of atoms vs mass number) for an hcl sample.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
(7 pts) hydrogen and chlorine atoms react to form simple diatomic molecules in a 1: 1 ratio that is...
Questions

History, 21.04.2021 19:20



Mathematics, 21.04.2021 19:20


History, 21.04.2021 19:20





Mathematics, 21.04.2021 19:20



Biology, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20



Mathematics, 21.04.2021 19:20