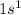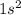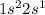Chemistry, 09.07.2019 12:00 loravillanueva87
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a. the 1s orbital in h is more stable than the 1s orbital in he+ b. the 2s orbital in he atom is less stable than the 2s orbital in he+ c. the 2s subshell in li is more stable than the 2p subshell in li?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

You know the right answer?
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a....
Questions

Mathematics, 07.04.2021 06:20

Social Studies, 07.04.2021 06:20

Mathematics, 07.04.2021 06:20

Physics, 07.04.2021 06:20

Mathematics, 07.04.2021 06:20


History, 07.04.2021 06:20





Biology, 07.04.2021 06:20






Mathematics, 07.04.2021 06:20

Mathematics, 07.04.2021 06:20