
Chemistry, 09.07.2019 11:30 taterbug3859
Acompound is 39.97% carbon, 13.41% hydrogen, and 46.62% nitrogen. what is the empirical formula? ch4n ch2n c2h8n2 c2h4n (the numbers are subscripts)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
Acompound is 39.97% carbon, 13.41% hydrogen, and 46.62% nitrogen. what is the empirical formula? c...
Questions


Computers and Technology, 17.03.2022 02:00

Mathematics, 17.03.2022 02:00

Computers and Technology, 17.03.2022 02:00



Social Studies, 17.03.2022 02:10


Mathematics, 17.03.2022 02:10




Mathematics, 17.03.2022 02:10



Mathematics, 17.03.2022 02:10


English, 17.03.2022 02:10

Spanish, 17.03.2022 02:20

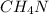 .
.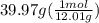 = 3.33
= 3.33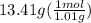 = 13.3
= 13.3 = 3.33
= 3.33 = 1
= 1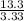 = 4
= 4

